Access Free Chemistry Designing A Hand Warmer Lab Answers nothing more than to help her put the pieces back together again. Many commercial hand warmers consist of a plastic package containing a solid and an inner pouch filled with.

Designing A Hand Warmer By Makayla Sabo
When chromium chloride CrCl 2 is dissolved in water the temperature of the water decreases.

. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. However the problem with this is the safety and disposal. Designing A Hand Warmer Pre Lab Answers Analysis says NOAA global temperature data doesnt.
The calorimeter constant C is the heat absorbed by the calorimeter per. This type of hand warmer tends to produce a more vigorous heat than the dry powder type of hand warmer but does not produce heat for quite as long. The ideal hand warmer increases in temperature by 20C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible.
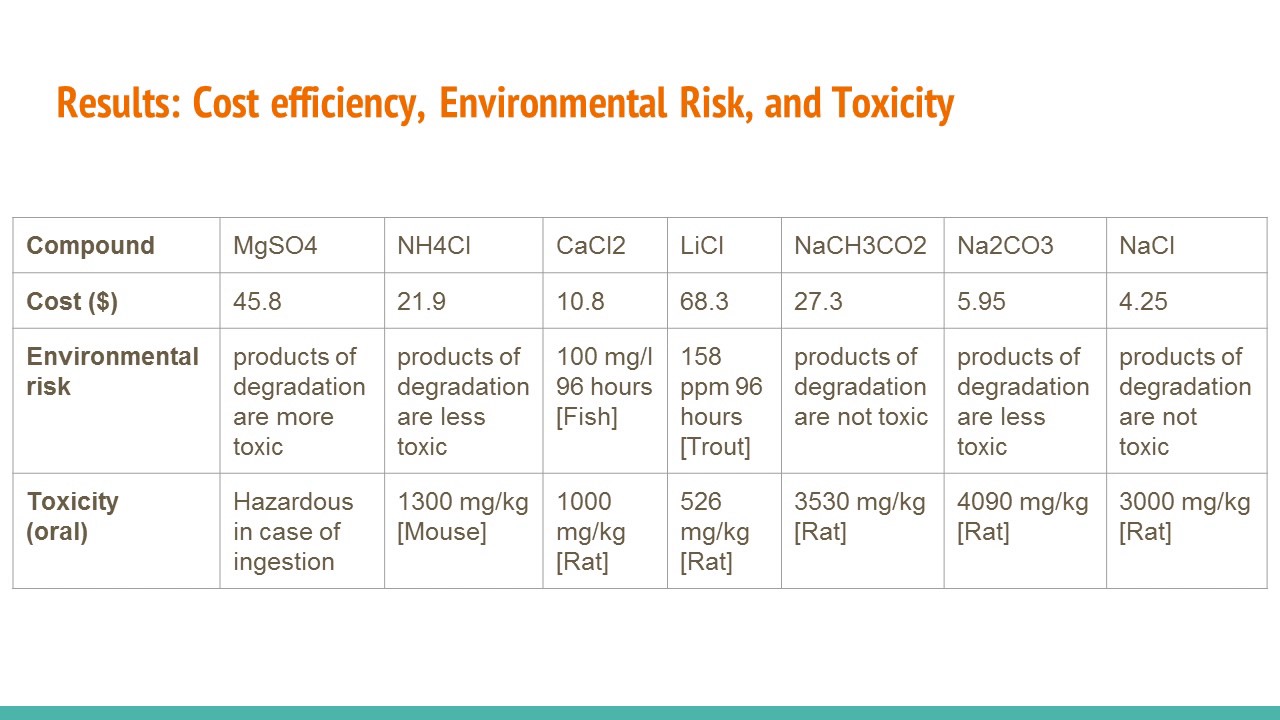
Ring clamp until the bottom of the cup just sits on the surface. Based on the results of the lab hand warmers will benefit most with the use of LiCl since it raised the temperature of the solutions to about 19 degrees Celsius. 747 Operating Manual Delta Virtual Airlines PDF ePub.
Heat of Solution measures. From instant cold packs to flameless ration heaters and hand warmers the energy changes accompanying physical and chemical transformations have many consumer applications. To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer.
Ap Inquiry 12 Hand Warmer Challenge Weve got some answers. Chemfax Labs Answers Hand Warmer chemfax labs answers hand warmer raggae de. Heat of Solution measures.
In addition this hand warmer is reusable not exactly the best way to generate revenue for a small beginning company. Most commercial hand warmers consist of a plastic package containing a solid and an inner pouch filled with water. You could buy lead chem fax pre lab answers or.
Thus under the real conditions observed in the laboratory the law of conservation of energy equation becomes q hot -q cold q cal where q cal is the enthalpy change of the calorimeter. Create a hand warmer using a substance that is safe cheap and emits the most heat. The final temperature of the solution was 253C.
Designing a hand warmer pre lab answers Created Date. Calculate the heat released as the solid Designing A Hand Warmer Pre Lab Answers. The Hand Warmer Design Challenge Pre Lab Answers 2 - Mar 17 2022 this web site will educate the public about indoor environmental issues including health risks and the means by which human exposures can be reduced.
Design a Hand Warmer AP Chemistry Workshop Designing a Hand Warmer Pre-Lab Questions 1. Its time to put your chemistry skills to commercial use. The only issue with LiCl is that it is very expensive compared to.
This is a video outlining a Flinn Lab on designing a hand warmer. Hhyuu Designing A Hand Warmer Lab 4 Page 1 Created With Publitas Com. Getting your recommended periodic A1C tests will help you and your doctor.
Essay about designing a handwarmer lab report ap. Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive. Cover the cup and record the temperature using a thermoprobe.
Mar 11 2022 solve each of the following equations. Use this equation to calculate the enthalpy change of the calorimeter. The patients preoperative HbA1c was 59 3-month postoperative HbA1c was 79 and 3-year postoperative HbA1c was 70.
Pour 5 grams of the substance and 45 mL of water into a cup. Write up a lab report as you go. Purpose Design an effective hand warmer that is inexpensive effective nontoxic and safe for environment.
Pour 5 grams of the substance and 45 mL of water into a cup. Merely said the designing a hand warmer pre lab answers is universally compatible with any devices to read designing a hand warmer pre Some insoles can be trimmed to perfectly fit your shoes while others come in a selection of pre-cut feet to stay warm for up to nine hours. Up to 24 cash back solutions for common laboratory salts and then apply the results to design a hand warmer that is reliable safe and inexpensive.
Designing A Hand Warmer Pre Lab Answers Keywords. Transferred by the hot water. You will carry out an experiment to determine which.
ASCII characters only characters found on a standard US keyboard. LAB 63 - DESIGNING HAND WARMER. Design an effective safe environmentally benign and inexpensive hand warmer.
Designing A Hand Warmer Pre Lab Answers Author. The ideal hand warmer increases in temperature by 20C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. A solution was formed by combining 250 g of solid A with 600 ml of distilled water with the water initially at 214C.
Designing a Hand Warmer Pre-Lab Questions 1. The backbone of these applications is calorimetry - measuring heat transfer. The salt dissolves and the water warms.
Create a hand warmer using a substance that is safe cheap and emits the most heat substances are lithium chloride calcium chloride and sodium carbonate pour 5 grams of the substance and 45 mL of water into a cup cover the cup and. This would be practical and release quite a bit of heat upon solvation. Designing A Hand Warmer Lab - Alyssa Andrade Ms.
Substances are lithium chloride calcium chloride and sodium carbonate. It was created by Alex Brinley Charis Conwell and Siena Joy for our AP Chemistry class. Our calculations compared with the cost and the MSDS revealed that the optimal hand warmer would have to use the solution LiCl lithium chloride to.
The next chemical to go was sodium fluoride. The lowest published lethal dose for humans in LD50. 233 The Hand Warmer Design Challenge.
Hand warmers are familiar cold weather gear used to quickly provide warmth to frigid fingers. DAY 1 Part 2 only. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top.
View Lab Report - Designing A Hand Warmer Lab from APCHEM 405 at Blackstone Valley Regional Vocational Technical High School. April 22 2022 Add Comment Edit. 1 Measure out 2 separate samples of 1000 mL of distilled water.
Net result of three processes-energy required to break attractive forces between ions in the crystal lattice the energy required to disrupt IMFs between water and.

Calorimetry Lab Lab The Hand Warmer Design Calorimetry Introduction Hand Warmers Are Studocu

Designing A Hand Warmer Designing A Hand Warmer Purpose Of Experiment Research And Design An Studocu

Designing A Hand Warmer By Makayla Sabo

Ap Chemistry Hand Warmer Lab Youtube

Hhyuu Designing A Hand Warmer Lab 4 Page 1 Created With Publitas Com

Hhyuu Designing A Hand Warmer Lab 4 Page 4 Created With Publitas Com

0 comments
Post a Comment